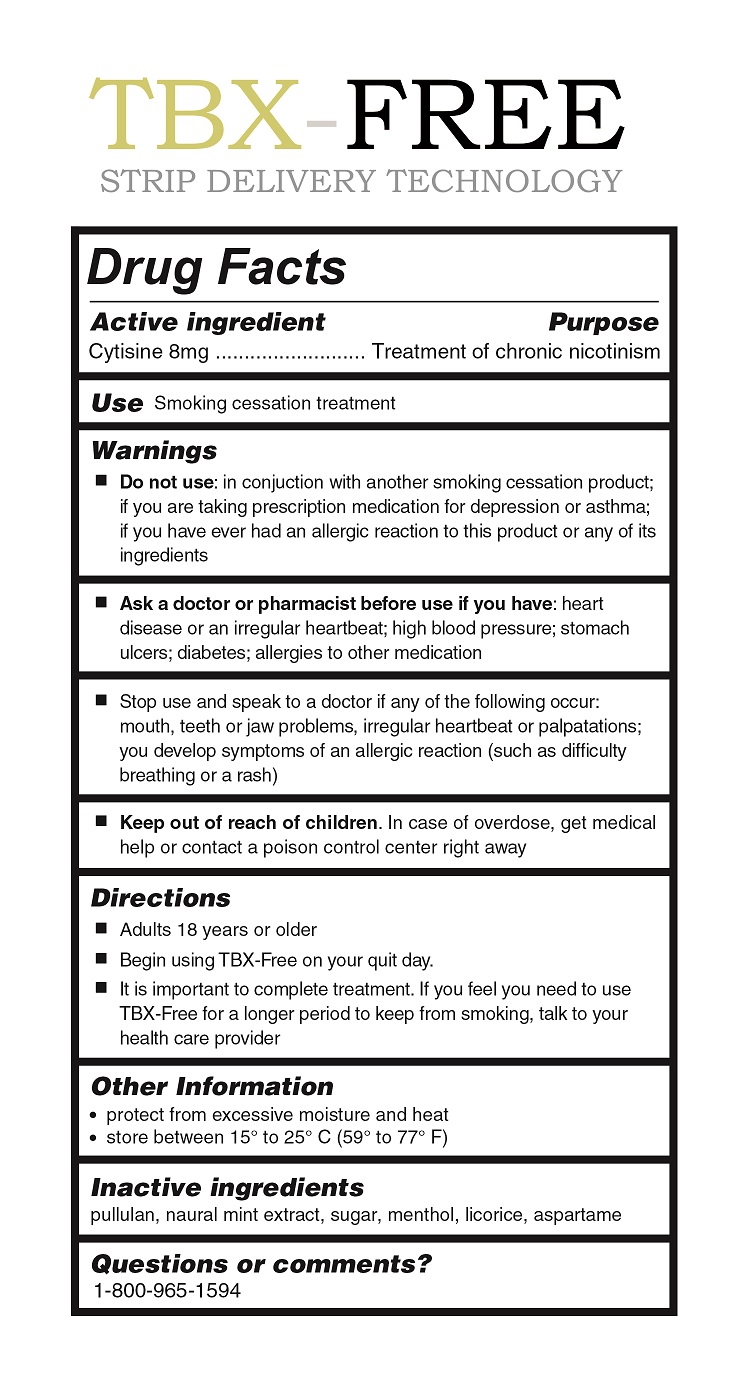
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Updated November 20, 2015
If you are a consumer or patient please visit this version.
KEEP OUT OF REACH OF CHILDREN In case of overdose, get medical help or contact a poison control center right away.
UseDIRECTIONS Adults 18 years or over Begin using TBX-Free on your quit day It is important to complete treatment. If you feel you need to use TBX-Free to keep from smoking, talk to your health care provider.
Warnings
Do not use: in conjunction with another smoking cessation product; if you are taking prescription medication for depression or asthma; if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor or pharmacist before use if you have: heart disease or an irregular heartbeat; high blood pressure; stomach ulcers; diabetes; allergies to other medication
Stop use and speak to a doctor if any of the following occur: mouth, teeth, or jaw problems, irregular heartbeat or palpitations; you develop symptoms of an allergic reaction (such as difficulty breathing or a rash)
OTHER SAETY INFORMATION Protect from excessiv moisture and heat Store between 15º and 25º C (59º to 77 º F) QUESTIONS OR COMMENTS 1-800-965-1594

| Product Information | |||
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC:69461-001 |
| Route of Administration | ORAL | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| CYTISINE (UNII: 53S5U404NU) (CYTISINE - UNII:53S5U404NU) | CYTISINE | 8 mg in 1 mg |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| SUCROSE (UNII: C151H8M554) | |
| MENTHOL (UNII: L7T10EIP3A) | |
| LICORICE (UNII: 61ZBX54883) | |
| PULLULAN (UNII: 8ZQ0AYU1TT) | |
| MINT (UNII: FV98Z8GITP) | |
| ASPARTAME (UNII: Z0H242BBR1) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | NDC:69461-001-01 | 110 mg in 1 BOX; Type 0: Not a Combination Product | 11/20/2015 | |
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| unapproved homeopathic | 11/20/2015 | ||
| Labeler - Dalian Jixin Electronic Information Co., Ltd. (544903951) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dalian Jixin Electronic Information Co., Ltd. | 544903951 | manufacture(69461-001) | |